
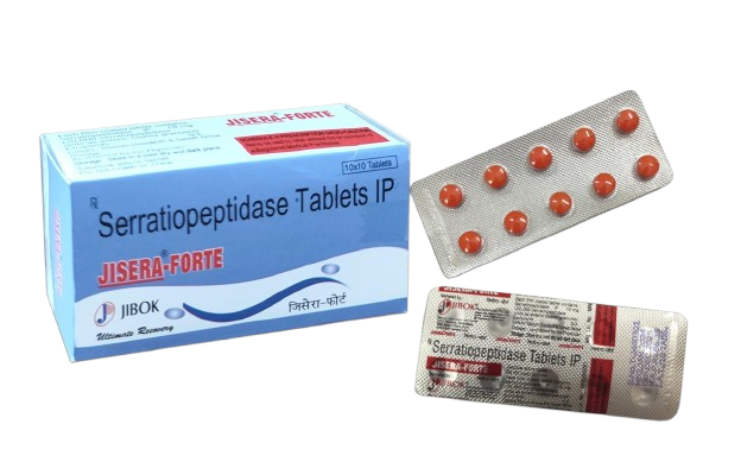
JISERA FORTE ™ Tablets Composition:-Each film coated tablet contains Serratiopeptidase 10 mg. (20,000 Serratiopeptidase units as enteric coated granules) Descriptions: - Improves the circulation at the inflammatory focus by breaking down abnormal exudates and protein and by promoting the absorption of the decomposed products through the blood and lymphatic vessels. Accelerates the elimination of sputum, pus and hematoma by breaking down and liquefying mucous secretions and fibrin clots. Indication: - Inflammation after operation and traumatic injury. As concomitant therapy for elimination of inflammatory edema and swelling in the following diseases Otorhinolaryngology.......................... Sinusitis Gynecology.................................. ... Engorgement of the breast Urology........................................... Cystitis, epididymitis Dentistry & Oral surgery.................... Pericoronitis of the wisdom tooth, alveolar abscess - Inadequate expectoration of sputum in the following diseases Bronchitis, bronchial asthma, and concomitant therapy with antitubercular agents in pulmonary tuberculosis. - Inadequate expectoration of sputum after anesthesia. Dosage: -Usually, for adults, one tablet orally, three times daily before meal without chewing. Dosage may be increased according to condition. Side effects: - The drug has certain side effects, that can affect individuals in different ways. The following are some of the side effects, that are often associated with the drug: Anorexia, Gastric Discomfort, Nausea Contraindications: - Patients with a history of hypersensitivity to the ingredients of this drug. Precautions:-In patients with blood coagulation abnormalities, severe hepatic and renal disturbances or under treatment with anti-coagulants, JISERA FORTE should be given under careful observation. JISERA FORTE may occasionally cause gastrointestinal disturbances such as anorexia, gastric discomfort and nausea. Administration should be discontinued when hypersensitive symptoms, such as skin eruptions. Drug Interactions: - There is no specific data to be described. Adverse Drug Reactions: - Skin: Stevens-Johnson syndrome, Toxic epidermal necrolysis, Rash, Pruritus Hypersensitivity: Shock, Anaphylactic symptoms Hepatic: Hepatitis, Jaundice Presentation: - JISERA FORTE tablets are available in blister strip of 10's. 10 such strips in a carton. Disclaimer: The above information is for general understanding of the visitor. Please consult a registered medical practitioner before taking the aforesaid medicine.
View Details


JIBRA™ D Capsules Composition: Each JIBRA™D hard gelatin capsule contains: Rabiprazole Sodium I.P. 20mg. (As enteric coated pellets) Domperidone B.P. 30mg. (As sustained release pellets) Description: Rabeprazole is a proton pump inhibitor and belongs to the class of antisecretory compounds (substituted benzimidazole), commonly known as ‘PROTON PUMP’ and Domperidone is a potent prokinetic with anti-emetic action. Indications: JIBRA™D is indicated for the relief of symptoms of 1. Dyspepsia 2. GERD 3. Nausea associated with acid peptic disorders 4. Post-operative nausea and vomiting 5. Chronic gastritis Dosage: JIBRA™D One capsule once daily. OR as directed by the physician. Contraindications: Contraindicated in individuals with known hypersensitivity to either of the active ingredients, and substituted benzimidazoles. Side effects: In general, JIBRA™D is well tolerated. However, few transient effects such as skin rash, allergic reactions, galactorrhoea, conjunctivitis etc. may be observed. Drug interactions: Rabeprazole : pH dependent interactions with digoxin and ketoconazole. Domperidone : (a). Concomitant administration of anticholinergic drugs may decrease the effect of domperidone. (b). Azole antifungals / macrolide antibiotic , increases plama levels of domperidone . Precautions: · Not recommended for continuous use or routine prophylaxis · Should be used with caution in patients with risk of arrhythmias · Pregnancy - Use not recommended in pregnancy. · Nursing Mothers - It is not known whether JIBRA™D is distributed into human milk, however, decision should be made to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother. · Pediatric Use - The safety and efficacy of JIBRA™D has not been established in children. Presentation: JIBRA™D are available in strip of 10's. 10 such strips in a carton. Disclaimer: The above information is for general understanding of the visitor. Please consult a registered medical practitioner before taking the aforesaid medicine.
View Details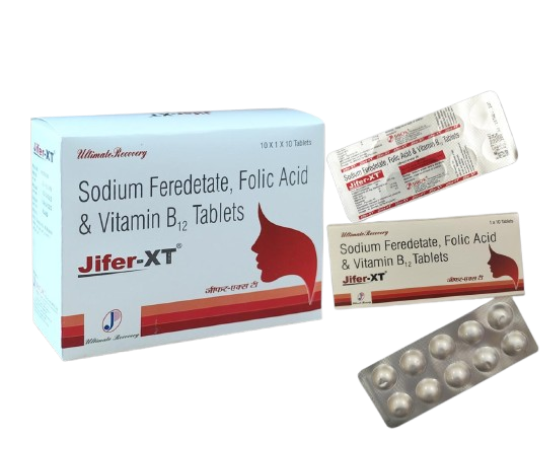
JIFER-XT ™ Tablets Composition:-Each film coated tablet contains Sodium Feredetate 231mg, Folic Acid 1.5mg & Vitamin B12 15 mcg. Descriptions: - Sodium feredetate is an iron preparation. Iron is a vital component of haemoglobin (oxygen-carrying pigment of red blood cells) and is therefore important in the formation of red blood cells. It is also a component of myoglobin, a pigment which stores oxygen in muscles for use during exercise. Iron is an essential component of several enzymes and is involved in the uptake of oxygen by the cells and the conversion of blood sugar to energy. This preparation is not an iron salt. It is an unionised form. Because of this, it is not astringent and does not discolour teeth. Sodium feredetate breaks down within the gastrointestinal tract to release elemental iron, which is then absorbed. Indication: - Iron deficiency Anaemia Pregnancy & lactation Post-surgery Dosage: - Usually, for adults, one tablet orally, 1 to 2 times daily after meal without chewing. Dosage may be increased according to condition. Side effects: - Medicines and their possible side effects can affect individual people in different ways. The following are some of the side effects that are known to be associated with this medicine. Because a side effect is stated here, it does not mean that all people using this medicine will experience that or any side effect. · Diarrhoea · Nausea Contraindications: -None known Drug Interactions: - Tetracyclines. Precautions:- None known Presentation: - Jifer-xt tablets are available in blister strip of 10's in a mono carton. Disclaimer: The above information is for general understanding of the visitor. Please consult a registered medical practitioner before taking the aforesaid medicine.
View Details

ALMOST ™ Tablets Composition:-Each uncoated tablet contains- Levocetirizine dihydrochloride 5mg. Descriptions:-Selective, long acting peripheral H1 receptor antagonist antihistamine. The increased polarity of Levocetirizine may decrease distribution of the drug into the CNS, resulting in reduce potential for adverse CNS effects. Tolerance does not occur. As it does not readily cross the blood brain barrier, it does not cause sedation or interfere with mental alertness or memory function. Indication: - Seasonal allergic rhinitis, perennial allergic rhinitis, ocular symptom (redness, lacrimation and itching), chronic idiopathic urticaria, urticaria dermographism, atopic eczema and contract dermatitis, also in acute allergic reactions due to drugs, food and insect bites. Dosage: - Adult and children above 6 years: 5 mg single intake orally. Children below 6 years: No adjusted dose. Elderly: Adjust dose on the basis of renal function. Pregnancy: Use with caution. Lactation: Use with caution as secreted in breast milk. Side effects: - All medicines may cause side effects, but many people have no, or minor, side effects. Check with your doctor if any of these most COMMON side effects persist or become bothersome when using Levocetirizine: Drowsiness; dry mouth; sore throat; tiredness. Seek medical attention right away if any of these SEVERE side effects occur when using Levocetirizine: Severe allergic reactions (rash; hives; itching; difficulty breathing; tightness in the chest; swelling of the mouth, face, lips, or tongue; unusual hoarseness); dark urine; fainting; fever; irregular heartbeat; mental or mood changes (eg, aggression, agitation); seizure; severe or persistent dizziness; shortness of breath; yellowing of the eyes or skin. Contraindications:- Hypersensitivity to levocetirizine, cetirizine, or its parent compound hydroxyzine. Patients with severe renal impairment (<10 ml/min creatinine clearance) should not be administered Almost tablet. Patients with rare hereditary problems of galactose intolerance, fructose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine. Drug Interactions: - In vitro data indicate that levocetirizine is unlikely to produce pharmacokinetic interactions through inhibition or induction of liver drug-metabolizing enzymes. No in vivo drug-drug interaction studies have been performed with levocetirizine. Drug interaction studies have been performed with racemic cetirizine. Antipyrine, Azithromycin, Cimetidine, Erythromycin, Ketoconazole, Theophylline, and Pseudoephedrine Pharmacokinetic interaction studies performed with racemic cetirizine demonstrated that cetirizine did not interact with antipyrine, pseudoephedrine, erythromycin, azithromycin, ketoconazole, and cimetidine. There was a small decrease (~16%) in the clearance of cetirizine caused by a 400 mg dose of theophylline. It is possible that higher theophylline doses could have a greater effect. Ritonavir Ritonavir increased the plasma AUC of cetirizine by about 42% accompanied by an increase in half-life (53%) and a decrease in clearance (29%) of cetirizine. The disposition of ritonavir was not altered by concomitant cetirizine administration. Warnings and precautions:-Precaution is recommended with the intake of alcohol and in those who are on central nervous system (CNS) depressants. Avoid engaging in hazardous occupations requiring complete mental alertness, such as driving or operatingmachinery, when taking levocetirizine. Presentation:- ALMOST tablets are available in blister strip of 10's. 10 such strips in a carton. Disclaimer: The above information is for general understanding of the visitor. Please consult a registered medical practitioner before taking the aforesaid medicine.
View Details
JIBRA™ 20 Tablets Composition: Each JIBRA™20 enteric coated tablet contains - Rabiprazole Sodium I.P. 20 mg. Description: JIBRA™20 is used to treat certain conditions in which there is too much acid in the stomach. It is used to treat duodenal ulcers and gastroesophageal reflux disease (GERD), a condition in which the acid in the stomach washes back up into the esophagus. Rabeprazole is also used to treat Zollinger-Ellison syndrome, a condition in which the stomach produces too much acid. Sometimes rabeprazole is used along with antibiotics to treat ulcers associated with infections caused by the H. pylori bacteria (germ) .Rabeprazole works by decreasing the amount of acid produced by the stomach. Indications: JIBRA™20 for short-term treatment in healing and symptomatic relief of duodenal ulcers and erosive or ulcerative gastroesophageal reflux disease (GERD); maintaining healing and reducing relapse rates of heartburn symptoms in patients with GERD; treatment of daytime and nighttime heartburn and other symptoms associated with GERD; long-term treatment of pathological hypersecretory conditions, including Zollinger-Ellison syndrome and in combination with amoxicillin and clarithromycin to eradicate Helicobacter pylori. Gastric ulcer Peptic ulcer disease (PUD) Maintenance of healing of erosive or ulcerative GERD Healing of erosive and ulcerative GERD Healing of duodenal ulcers. Treatment of symptomatic GERD Treatment of pathological hypersecretory conditions (Zollinger-Ellison syndrome) Helicobacter pylori eradication to reduce risk of duodenal ulcer recurrence Dosage: When recommending a rabeprazole dosage, your doctor will take several factors into consideration, including the condition you are being treated for and any other medications you may be taking. The recommended dose with rabeprazole for people with symptoms of gastroesophageal reflux disease (GERD) is 20 mg once a day for four weeks. The recommended starting dosage for people with excess acid production is rabeprazole 60 mg a day. Your healthcare provider may choose to increase the rabeprazole dosage if symptoms continue or decrease the dose if side effects occur. Contraindications: hypersensitivity to rabeprazole, substituted benzimidazoles or any of components of its pharmaceutical forms. pregnancy: FDA Pregnancy Ratings: B lactation: Thomson Lactation Ratings: Infant risk cannot be ruled out. Side effects: Rabeprazole adverse reactions/side effects include: In clinical trials the most common side effect assessed as possibly or probably related to AcipHex was headache in 2.4% of patients vs 1.6% taking placebo. diarrhea vomiting nausea abdominal pains constipation meteorism dry mouth increased or decreased appetite asthenia headache anxiety sleeplessness vertigo thrombocytopenia granulocytopenia leukocytopenia skin eruption erythema myalgia arthralgia muscle or bone pain Drug interactions: Rabeprazole decreases the concentration of ketoconazole in the plasma (in 33%), increases the concentration of digoxin (in 22%), and does not interact with liquid antacids. Rabeprazole is compatible with any medicine metabolized by the CYP450 (theophylline, warfarin, diazepam, phenytoin). Precaution: Before taking rabeprazole, tell your doctor and pharmacist if you are allergic to rabeprazole, lansoprazole, omeprazole, pantoprazole or any other medications. tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products you are taking. Be sure to mention any of the following: cyclosporine, clopidogrel, digoxin, and ketoconazole. Your doctor may need to change the doses of your medications or monitor you carefully for side effects. tell your doctor if you have or have ever had liver disease. tell your doctor if you are pregnant, plan to become pregnant, or are breast-feeding. If you become pregnant while taking rabeprazole, call your doctor. Presentation: JIBRA™20 tablets are available in alu-alu strip of 10's. 10 such strips in a carton. Disclaimer: The above information is for general understanding of the visitor. Please consult a registered medical practitioner before taking the aforesaid medicine.
View Details
JIOX ™ OZ Tablets Composition: Each film coated tablet contains : Ofloxacin U.S.P. 200 mg. Ornidazole 500 mg. Description: Mixed aerobic and anaerobic infections are quite common in lower respiratory tract infections, diabetic foot infections, urinary tract infections, post surgical procedures, in acute diarrhoea and dysentery. Ofloxacin is one of the fluorinated quinolones with broad-spectrum coverage against gram-negative bacteria, gram-positive bacteria and also some anaerobes, whereas, Ornidazole is a 5-nitroimidazole derivative having broad-spectrum antimicrobial activity against gram-positive and gram-negative anaerobic bacteria and additionally broad-spectrum coverage against protozoas. Combining the two, results in a true broad-spectrum activity, to treat systemic infections of mixed aerobic/anaerobic etiology, in all situations where the time is critical and laboratory investigations are not feasible. Indications: 1. Oral therapy of moderate to severe systemic infections with a probability of having mixed aerobic and anaerobic bacterial infections. In some cases, cephalosporins and/or aminoglycosides may be co-prescribed wherever necessary. These infections include : GI infections like appendicitis, cholecystitis. Post-operative infections after abdominal/gynaecological operations. Lung infections like bronchiectasis, lung abscess, empyema, etc. Acute or chronic bone and joint infections. Infections in patients with Diabetes Mellitus. Infections in immunocompromised patients. Oral follow-up of parenteral therapy with Ofloxacin and Ornidazole or Tinidazole. 2. Acute diarrhoea or dysentery due to bacterial, protozoal or mixed causes. Dosage: Adults: 1 JIOX-OZ™ tablet twice daily after food for 5 days or as directed by the Physician. Contraindications: Known hypersensitivity to either ingredients. Severe renal insufficiency. Due to inadequate safety data, the drug should not be used during pregnancy, lactation and childhood, except where the risk to benefit ratio is justified. Side Effects: The side effects of the tablet could be due to the individual ingredients. These include the following: Ornidazole • Slight metallic taste, nausea, vomiting and gastrointestinal disturbances. • Urticaria, angioneurotic edema and other allergic states. • Dizziness, drowsiness, headache, ataxia. • Darkening of urine. • Rare instances of relapse of epileptiform seizures. Ofloxacin • Mild gastrointestinal upsets. • Rare cases of skin rashes, dizziness and headache. Precautions: Use with caution in mild to moderate renal failure, after appropriate dosage adjustment. Avoid alcohol during therapy and for two days after, in order to avoid risk of antabuse reaction. Ornidazole may augment the activity of oral anticoagulants, hence the dose of the latter may need reduction. Presentation: JIOX-OZ™ tablets are available in blister strip of 10's. 10 such strips in a carton. Disclaimer: The above information is for general understanding of the visitor. Please consult a registered medical practitioner before taking the aforesaid medicine.
View Details
JICALVIT ™ Tablets Composition: Each film-coated tablet contains 1.25 gm of Calcium carbonate from an organic source (Oyster Shell) equivalent to Elemental Calcium 500mg IP and Vitamin D3 250 i.u. Description: Calcium plays a critical role in the body. It is essential for normal functioning of nerves, cells, muscle and bone. Calcium prevents bone loss and is associated with a modest reduction in fracture risk. Calcium and vitamin D preparations are used to prevent or to treat calcium deficiency. Indications: Supplement in pregnancy, lactation, osteoporosis, osteomalacia, rickets and other calcium deficiency states Dosage: 1 tablet 2-3 times a day or as recommended by the physician Contraindications: No known contraindications. Side effects: All medicines may cause side effects, but many people have no, or minor, side effects. No COMMON side effects have been reported with Calcium Carbonate with Vitamin D . Seek medical attention right away if any of these SEVERE side effects occur when using Calcium Carbonate with Vitamin D: Severe allergic reactions (rash; hives; itching; difficulty breathing; tightness in the chest; swelling of the mouth, face, lips, or tongue); loss of appetite; nausea; severe or persistent constipation; vomiting. Drug interaction: Calcium carbonate can make it harder for your body to absorb other medications you take by mouth. Tell your doctor if you are taking digoxin , antacids, other calcium supplements; calcitriol, vitamin D supplements; doxycycline , minocycline or tetracycline. This list is not complete and other drugs may interact with calcium carbonate. Tell your doctor about all medications you use. This includes prescription, over-the-counter, vitamin, and herbal products. Do not start a new medication without telling your doctor. Precaution: Follow your healthcare provider's instructions about any restrictions on food, beverages, or activity. Presentation: JICALVIT™ tablets are available in blister strip of 10's. 10 such strips in a carton. Disclaimer: The above information is for general understanding of the visitor. Please consult a registered medical practitioner before taking the aforesaid medicine.
View Details

Undenatured type II collagen, Calcium, Cholecalciferol, Vitamin C , Magnesium, Zinc, Copper & Manganese tablets.
View Details
JIMETH PLUS ™ CAPSULES Composition: - Each film coated hard gelatin capsule contains: Methylcobalamin -1500 mcg, Alapha Lipoic Acid -100mg, Folic acid – 1.5mg,Thiamine Mononitrate -10 mg.Pyridoxine HCL - 3 mg. Descriptions: - Cobalamin is a water soluble vitamin. It maintains structure and functions of neurons. The powerful Neurotropic combination of Methylcobalamine with Pyridoxine HCL, Folic acid & Thiamine Mononitrate will provide excellent result in Neurological impairment.Amongst the Neuropathy patients nerve damage is more with diabetic population. Alpha Lipoic Acid is known to offer more beneficial effects on Diabetic Neuropathy as Alpha Lipoic Acid has been shown to prevent nerve dysfunction in diabetic patients. Indication: - Sleep disorders Diabetic Neuropathy Peripheral Neuropathy Atherosclerosis Hyper Homocysteinemia Rheumatoid Arthritis Multiple Sclerosis Dosage: - Once daily Side effects: - Dermatologic: Itching, rash, transitory exanthema, and urticaria ,Gastrointestinal: Diarrhea, Hematologic: Peripheral vascular thrombosis, Treatment of vitamin B12 deficiency can unmask polycythemia Vera, which is characterized by an increase in blood volume and the number of red blood cells. Contraindications: - Leber’s disease: Vitamin-B12 in the form of cyanocobalamin is contraindicated in early Leber's disease, which is hereditary optic nerve atrophy. Vitamin-B12 can cause severe and swift optic atrophy. Drug Interactions: - Alcohol, Amino salicylic acid, metronidazole decrease gastrointestinal (GI) absorption of vitamin B12,Colestipol, Cholestyramine, Metformin ,Neomycin, Nicotine, Nitrous oxide, Phenytoin , Phenobarbital, primidone Proton pump inhibitors, Zidovudine. Precautions: - None knownPresentation: - JIMETH PLUS capsule are available in Alu-Alu strip of 10's.10 such strips in a carton.Disclaimer: The above information is for general understanding of the visitor. Please consult a registered medical practitioner before taking the aforesaid medicine.
View Details
JIPAN ™ D TabletsComposition: Each enteric coated tablet contains Pantoprazole Sodium Sesquihydrate equivalent to Pantoprazole 40 mg and Domeperidone 10 mg Description: JIPAN™-D is a combination of pantoprazole and domperidone. Pantoprazole is a potent proton pump inhibitor. It inhibits the H + /K + - ATPase enzyme (proton pump) irreversibly and blocks the final step of acid secretion. Domperidone is a potent dopamine receptor (D2) antagonist. It increases motility of GI tract by inhibiting the action of dopamine and fastens gastric emptying. Domperidone increases Lower esophageal sphincter pressure and prevents reflux of stomach content into esophagus. Domperidone also inhibits the D2 receptor in the chemoreceptor trigger zone thereby it prevents nausea and vomiting. Indications: Combination of Pantoprazole and Domperidone gives a complete symptomatic relief from Dyspepsia / Heartburn / Acid-Pepsin disorder associated with nausea, vomiting and epigastric pain / Chronic gastritis.Dosage: - Oral dose for adults is 10-20 mg every 4-8 hours. - Oral dose for children is 0.2-0.4 mg/kg every 4-8 hours. Rationale of Combination Therapy Hyperacidity and Hypomotility are common GIT problems and frequently co-exist together. Excess hydrochloric acid and defective clearance of the acid and other gastric content from the stomach together or individually give rise to nausea, vomiting and upper abdomen discomfort due to irritation of gastric mucosa. Pantoprazole suppresses acid secretion and Domperidone stimulats gastric motility. Both the drugs are devoid of any major side effects. Domperidone does not cross BBB, so no extrapyramidal syndrome Contraindications: In patients with known sensitivity or intolerance to any of the drugs. Domperidone should not be used whenever gastrointestinal stimulation might be dangerous, i.e., gastrointestinal hemorrhage or mechanical obstruction. Side effect: 1) Very rarely extrapyramidal syndrome - e.g. Parkinsonism, tardive dyskinesia.2) Domperidone may cause galactorrhoea and gynaecomastia due to Hyperprolactinaemia 3) Other minor side effects - Dry mouth, thirst - Headache - Nervousness - Diarrhoea - Skin rash, itching - Increase in TSH level, hypothyroid woman. Drug interaction: Domperidone may reduce Hypoprolactinaemic effect of BromocriptinePrecaution: Lactation. Pantoprazole: Not recommended in child <12 yrs. Long-term therapy may lead to bacterial overgrowth in the GIT. Domperidone: Increases serum prolactin levels resulting to galactorrhoea in females and gynaecomastia in males. Hypertensive crisis in patients with phaeochromocytoma.Presentation: JIPAN™-D tablets are available in alu-alu strip of 10's. 10 such strips in a carton. Disclaimer: The above information is for general understanding of the visitor. Please consult a registered medical practitioner before taking the aforesaid medicine.
View Details


Diacerien, Glucosamine Sulphate Potassium Chloride & Methyl Sulfonyl Methane Tablets.
View Details
JIDOL®AP Tablets Composition: Each JIDOL®-AP film coated tablet contain aceclofenac 100 mg and Paracetamol 500 mg Description: JIDOL®-AP is a combination of aceclofenac and paracetamol. Aceclofenac is an NSAID, It directly blocks PGE 2 secretion at the site of inflammation by inhibiting IL-beta and TNF in the inflammatory cells. Aceclofenac has been demonstrated to inhibit cyclooxygenase (COX) activity and to suppress the PGE 2 production by inflammatory cells. Paracetamol is an acetaminophen derivative with analgesic, antipyretic properties. Indications: JIDOL®-AP is indicated for the relief of symptoms of Acute musculoskeletal painful conditions in adults Low back pain Acute flares of RA & OA Painful and inflammatory ENT conditions (Pharyngitis & tonsillitis) Post-operative pain Gynaecological pain e.g. Dysmenorrhoea Dental pain Fractures Dosage: JIDOL®-AP tablets are supplied for oral administration in adults. The maximum recommended dose of JIDOL®-AP is two tablets daily, taken as one tablet in the morning and one in the evening. Contraindications: Aceclofenac should not be administered to patients hypersensitive to aceclofenac or other NSAIDs or patient with a history of aspirin or NSAIDs related allergic or anaphylactic reactions or with peptic ulcers or GI bleeding, moderate or severe renal impairment Side effects: Most of the adverse events are minor and reversible with treatment discontinuation. The majority of side effects are related to gastrointestinal system (dyspepsia, abdominal pain, nausea and diarrhea), most frequent being dyspepsia, abdominal pain and rise in hepatic enzymes. Other rare side-effects include dizziness, constipation, vomiting, ulcerative stomatitis, rash, dermatitis, headache, fatigue, allergic reactions, anemia, granulocytopenia, thrombocytopenia, neutropenia, oedema, palpitation, leg cramps, flushing, purpura, paraesthesia, tremors, gastrointestinal bleeding, gastrointestinal ulceration, pancreatitis, interstitial nephritis, depression, abnormal dreaming, somnolence, insomnia, vasculitis, hypoglycemia, rise in blood urea, serum creatinine and serum potassium. As with other NSAIDs, severe mucocutaneous skin reactions may also occur with JIDOL®-AP. Drug interaction: Drug interactions associated with Aceclofenac are similar to those observed with other NSAIDs. Aceclofenac may increase the plasma concentrations of lithium, digoxin and methotrexate. It may increase the activity of anticoagulants, inhibit the activity of diuretics, enhance cyclosporine nehrotoxicity and precipitate convulsions when co-administered with quinolone antibiotics. Co-administration of Aceclofenac with other NSAIDs and corticosteroids are to be avoided due to increased incidence of side-effects. The risk of Paracetamol toxicity may be increased in patients receiving other potentially hepatotoxic drugs or drugs that induce hepatic microsomal enzymes. Coadministration of Paracetamol with rifampicin, isoniazid, chloramphenicol, antiepileptic drugs and antiviral drugs is to be avoided. Metoclopromide may increase the absorption of Paracetamol whereas excretion and plasma concentration may be altered when coadministered with probenecid. Cholestyramine also reduces the absorption of Paracetamol Precautions: JIDOL®-AP may cause dizziness. Driving or operating machinery are to be avoided. Individuals receiving long-term treatment should be regularly monitored for renal function tests, liver function tests and blood counts. It is to be used with caution in hepatic porphyria, coagulation disorders, history of peptic ulcers, ulcerative colitis, Crohn’s disease, SLE, cerebrovascular bleeding, pregnancy and lactation. Caution should be exercised in patients with mild to moderate impairment of cardiac, hepatic or renal function and in elderly patients who are more likely to be suffering from these conditions. Caution is also required in patients on diuretic therapy or otherwise at risk of hypovolemia. Presentation: JIDOL®-AP tablets are available in blister strip of 10's. 10 such strips in a carton. Disclaimer: The above information is for general understanding of the visitor. Please consult a registered medical practitioner before taking the aforesaid medicine.
View Details
JIPAN ™ 40 TABLETS Composition: Each enteric coated tablet contains- Pantoprazole sodium sesquilhydrate eq. to Pantoprazole 40 mg. Description: Pantoprazole is used to treat certain conditions in which there is too much acid in the stomach. It is used to treat gastroesophageal reflux disease (GERD), a condition in which the acid in the stomach washes back up into the esophagus. This medicine may also be used to treat Zollinger-Ellison syndrome or other conditions (such as cancer) in which the stomach produces too much acid Pantoprazole works by decreasing the amount of acid produced by the stomach. Indication: Although pantoprazole is approved for the treatment of gastroesophageal reflux disease (GERD), like other PPI's it also is used for treating ulcers of the stomach and duodenum, and the Zollinger-Ellison Syndrome. Dosage: For GERD the recommended dose for adults is 40 mg daily for 4-8 weeks. It generally is recommended that tablets be taken approximately 30 minutes prior to meals for maximal effectiveness. Tablets should be swallowed whole and should not be crushed, split or chewed. Contraindications: Pantoprazole tablets are contraindicated in patients with known hypersensitivity to any component of the formulation. Side effects: Pantoprazole like other PPIs is well-tolerated. The most common side effects are diarrhea, nausea, vomiting, constipation, rash and headaches. Dizziness, nervousness, abnormal heartbeat, muscle pain, weakness, leg cramps and water retention rarely occur. Drug interaction: Pantoprazole is less likely than omeprazole to interact with other drugs. The absorption of certain drugs may be affected by stomach acidity, and, as a result, pantoprazole and other PPIs that reduce stomach acid also reduce the absorption and concentration in blood of ketoconazole (Nizoral) and increase the absorption and concentration in blood of digoxin (Lanoxin). This may lead to reduced effectiveness of ketoconazole or increased digoxin toxicity, respectively. Use of pantoprazole in pregnant women has not been adequately evaluated. Pantoprazole has not been studied in nursing women. Precaution: Before taking pantoprazole, tell your doctor or pharmacist if you are allergic to it; or to similar drugs (e.g., lansoprazole, omeprazole); or if you have any other allergies. Before using this medication, tell your doctor or pharmacist your medical history, especially of: severe liver disease, diabetes (poorly controlled), other stomach problems (e.g., tumors). Some symptoms may actually be signs of a more serious condition. Tell your doctor immediately if you have: heartburn combined with lightheadedness/sweating/dizziness, chest pain or shoulder/jaw pain (especially with trouble breathing), pain spreading to arms/neck/shoulders, unexplained weight loss. This medication should be used only when clearly needed during pregnancy. Discuss the risks and benefits with your doctor. It is not known whether this drug passes into breast milk. Breast-feeding while using this drug is not recommended. Consult your doctor before breast-feeding. Presentation: JIPAN™40 tablets are available in alu-alu strip of 10's. 10 such strips in a carton. Disclaimer: The above information is for general understanding of the visitor. Please consult a registered medical practitioner before taking the aforesaid medicine.
View Details
Jiaz ™ Tablets Compositions: JIAZ 500: Each film coated tablet contains- Azithromycin I.P. 500mg. Descriptions: The drug is an azalide antibiotic, belongs to macrolide group. It is more active against gram-negative organisms. It is absorbed from the GIT after oral administration. The blood concentration is low, while the anti-microbial activity is high. It is concentrated in leukocytes and penetrates the tissues well and has a long half life. Post antibiotic effect (PAE) is also very high. Indications: Azithromycin is used to treat bacterial infections in many different parts of the body. It can be used to treat Streptococcal infections of the throat, Chlamydial infections, M.pneumoniae, C.jejuni, H.Influenzae, bronchitis, skin infections etc. Dosage: For treating most types of common bacterial infections, the recommended dosage of azithromycin is 250 mg or 500 mg once daily for three to five days. Dosing for children can range (depending on body weight) from 5 mg to 20 mg per kilogram of body weight per day, once daily for three to five days. The recommended dose for treating sexually transmitted diseases (STD) is 1 g to 2 g, given one time only. Contra Indications: Contraindicated in hepatic and renal dysfunction, Pregnancy. Side effects: Most common side effects are gastrointestinal; diarrhea (5%), nausea (3%), abdominal pain (3%) and vomiting. Fewer than 1% of patients stop taking the drug due to side effects. Serious allergic reactions, nervousness, dermatologic reactions, and fatalities have been reported. As with all antimicrobial agents, pseudomembranous colitis can occur during and up to several weeks after azithromycin therapy. This drug may interfere with the effectiveness of birth control pills; other forms of contraception may be required during the treatment period. Azithromycin suspension has an objectionable taste, so can be difficult to administer to young children (i.e. 2 - 5 years) who may spit it out. Allergic reaction: Patients who suffer from an allergic reaction to azithromycin can experience blood in the stool 4–10 days after ingestion, although cases of this have been recorded as early as after the first day of ingestion. These allergies are usually non-severe if the treatment is immediately stopped. A severe reaction includes a severe rash, hives, breathing difficulties, or dizziness. Interactions: Antacids, aluminium and magnesium-containing: Antacids may decrease the amount of azithromycin in the blood, which may decrease its potency. Azithromycin should be taken at least 1 hour before or at least 2 hours after antacids. Precautions Hepatic disease : Patients with severe hepatic disease may have an increased risk of adverse effects. Presentation: JIAZ 500 tablets are available in blister strip of 3's in a mono carton. 10 such strips in a carton. Disclaimer: The above information is for general understanding of the visitor. Please consult a registered medical practitioner before taking the aforesaid medicine.
View Details